What we are doing by RQ and MA
RQ15 Distinguished Fellowship Award Lecture
Paul Hideo Shingu
Professor Emeritus Kyoto University
Introduction
Both RQ(rapid-quenching) and MA(mechanical-alloying) are useful methods to explore various meta-stable and unstable phases of materials. A brief over view of these technologies from the thermodynamic and mechanical points will be given here to help understanding what we are doing using such methods.
1 RQ is to create under-cooling
Ever since the ancient technology of making sharp and hard swords, rapid quenching has been a useful method to create meta-stable steel crystalline structure "martensite" which are otherwise temperature-wise hidden under the equilibrium "α" phase. In general, it must be remembered that there can be many meta-stable phases while the equilibrium phase (the most stable phase) is one.
The basic physical meaning of RQ (rapid quenching) is the creation of large under-cooling of materials avoiding the formation of the most stable phase at equilibrium temperature. The faster the quenching rate the less the time available for the nucleation of new phases so that the larger the under-cooling attainable. It also has to be remembered that under-cooled state also is one of the meta-stable phases(1,2).
Depending on what is the initial state of the material at high temperature from which RQ method is applied, RQ is called, solid-quenching, liquid-quenching and vapor-quenching.
Figure 1 shows the energetic view of the relation between the stable phase and meta-stable phases for the case of liquid-quenching. A schematic explanation of the energetic relation among stable, unstable and meta-stable states of a material is shown in Fig.2.
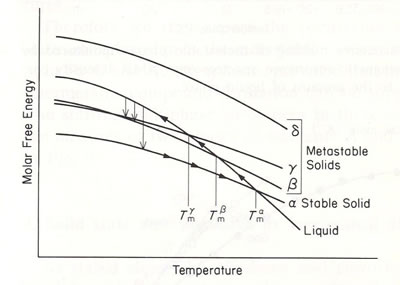
Figure 1
Schematic view of the energetic relations among the stable and meta-stable phases for the case of liquid under-cooling. The melting temperature of the stable solid phase is the highest and it is lower the less stable the meta-stable phase. In this figure, δ phase is not attainable by under-cooling.
2 The Ostwald's step rule favors the formation of meta-stable phase to the stable phase
Experimentally, emergence of meta-stable phases from under-cooled liquid is quite common. The Ostwald's step rule states that in general, the formation of meta-stable phase is favored rather than the most stable phase from the same initial phase when both phases are in the temperature range in which phase change is possible(3).
The "freezing rain" is one of the examples of Ostwald's step rule observable in nature. The under-cooled water vapor produces under-cooled water (rain drops) rather than the most stable snow flake(solid water) below freezing temperature (0℃) when there are not enough dust (nucleation sites) in the atmosphere for solid water formation.
The reason for this phenomenon is explained as due to the smaller interfacial energy between the meta-stable phase and the initial phase compared to that between the initial and stable phase. The structural difference between meta-stable phase and initial phase is smaller than the difference between the stable and initial phase. The values of interfacial energy generally are proportional to the structure difference of phases.
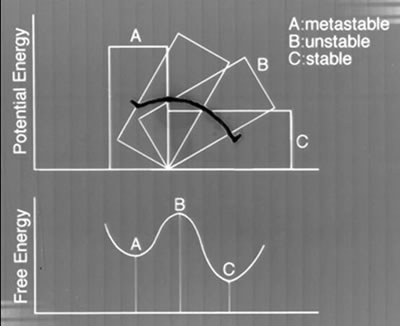
Figure 2
A schematic illustration explaining the energetic relationships among the stable, unstable and meta-stable phases. Free energy states of phases and potential energy relations of an object in gravitational field are compared.
3 MA may produce both meta-stable and non-stable state of materials
MA, mechanical alloying, is a general term meaning extensive pulverization of solid component or mixtures of components by mechanical repeated hammering or kneading.
In contrast to RQ, in the case of MA the processing temperature is usually around room temperature or below. At temperature range where kinetic motion of atoms are slow, the repeated kneading of powder mixture of two pure components may result in the formation of nano-order proximity of different elements. Such state is quite similar to the elemental mixtures in liquid or in vapor. So that without any rapid processing, one may obtain many meta-stable and/or unstable states of materials like these obtainable by RQ by MA as explained in Fig.3.
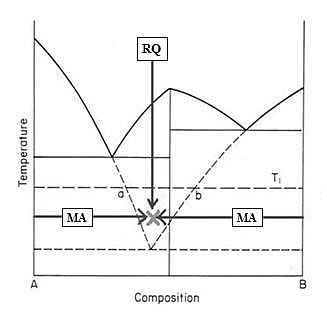
Figure 3
A schematic binary phase diagram in which the physical meaning of RQ and MA are shown by the arrows(2).
4 Energetic comparisons of various techniques of meta-stable phase exploration.
RQ and MA are two handy techniques to realize non-equilibrium structures but there are many other methods known to attain such states of materials. Each of such method has its own advantages and disadvantages technologically, but the most crucial point of the ability of the technique depends on the energetic measure how much free energy excess each processing methods can attain.
David Turnbull first showed the table of energetic potential of various rapid quenching methods in relation to the rapid quenching rate(4). Table1 shows an extension of such table arranged in terms of realizable free energy excess for wide range of applicable techniques(5).
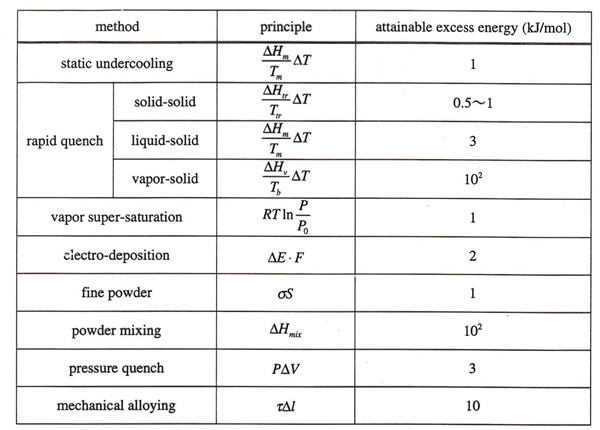

Table 1
Comparison of excess energy which may be put into the solid material by various methods
From Table1, one may notice the advantages of RQ and MA in realizing fairly large free energy excess in mass production bases.
5 Examples of nano-structure formation by MA
Regulated repetition of kneading is in principle the same technique as the making crusts of apple pies in bakery. This process is known as the fundamental way of creation of "chaos" states in various field of study. A schematic drawing of repeated kneading for metallic materials is shown in Fig.4.(6).
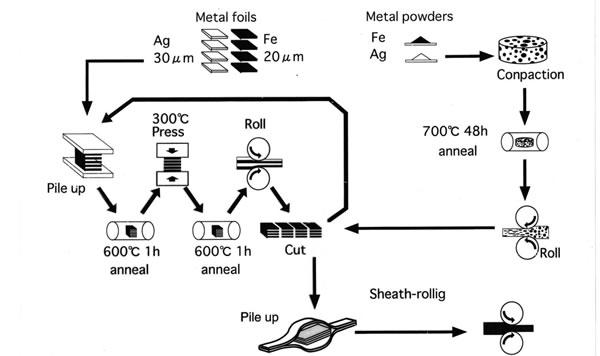
Figure 4
A schematic illustration of kneading applicable to metallic materials. In this case depending on whether the starting shape of materials are thin plates or mixture of powders, the sample obtained may be regular nano-layers or randomly mixed nano-grained sheets.
Fig. 5 shows a regular nano-layered structure formed in a Ag-Fe alloy using the procedure shown in Fig.4. (6). Ag and Fe are mutually strongly repulsive elements so that even in almost atomistic mixture of these elements, fairly regular layers can be formed by repeated kneading. One of the advantages of nano-layer formation by MA, in comparison with the alternative vapor deposition, is the number of layers can be as many as over millions of layers. As a result, the directional anisotropy of physical properties can be examined in both parallel and perpendicular direction to the layers as shown in Fig.6.
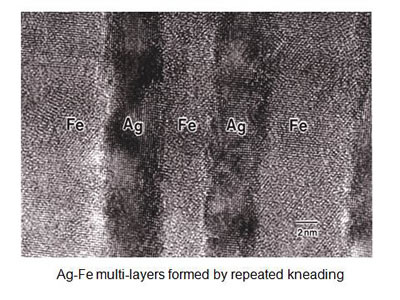
Figure 5
A nano-scale multi-layers of Ag and Fe formed by the repeated "Baker's Transformation"
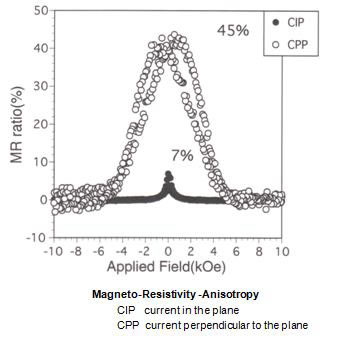
Figure 6
An example of magneto-resistivity anisotropy observed in a sample shown in Fig.5. It is to be noted that the magneto-resistivity change is much greater when the electrical current is perpendicular to the layers.
6 MA and Mechano-Chemistry
6.1 Low temperature iron smelting
Repeated mechanical crashing or mechanical grinding, the technique sometimes called as "Mechanical Milling" also produces nano-scaled fine powers for brittle materials. The enhancement of chemical reactions due to mechanical treatments are sometimes referred to as "Mechano-Chemistry". Close proximity of different elements in nano -scale may greatly enhance the chemical reaction rate of these elements.
As an example, the reduction of iron oxides by carbon which is a basis of iron smelting today can be enhanced greatly when the iron ore is mixed with carbon by mechanical grinding to the size of microns in diameter. The reduction temperature may become as low as around 1000℃which is close to the thermodynamically possible low temperature limit of the reduction reaction as shown in Fig.7..
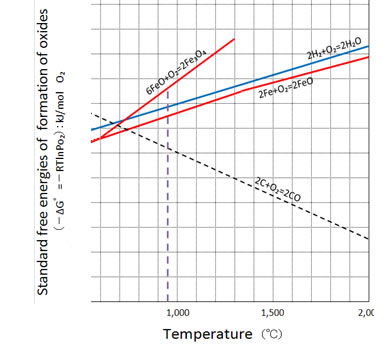
Figure 7
A part of the Ellingham Diagram related to the thermodynamic stabilities of iron oxides relative to carbon mono-oxide gas and to water vapor. It is readily understandable that iron oxide FeO (and hence Fe2O3) can be reduced by solid carbon in the temperature above around 750℃.
Fig.7 indicates that technologically iron-smelting can readily be possible at temperatures around 1000℃. However, due to the mass-scale industrial advantage, production of iron is usually performed by "blast furnace" in which the maximum heating temperature is around 1700℃.
In order to run a blast furnace, as the name suggests, large amount of air (or sometimes oxygen) is blown into the furnace partly for heating by burning carbon and partly to produce carbon mono-oxide for reduction of iron oxides. This technique is quite useful but when the whole process is reviewed in sober eyes, it may seem curious to provide oxygen for the purpose of reduction of iron-oxides.
When both iron ore and carbon are ground into the size of several microns to several tens of microns, the surface area of both reacting materials can be increased by tens of thousands times greater than the case when compared with the initial sizes which may be in centimeters. When mixed powders are packed closely, the reduction reaction may take place at temperature range as low as around or below 1000℃ and within very short reaction time. When the initial powder size can be reduced down to sub-micron size, almost instantaneous reduction may be possible at 1000℃。
Fig.8 shows an example of the iron smelting experiment from mechanically milled and compacted powder mixture of Fe2O3 and wood charcoal in stoichiometric composition(7).
It is to be noticed that pure iron in the form of micron-sized fibrous structure (called the sponge iron) are formed at 950℃in one hour. The iron thus made is pure iron due to the solid state reaction in which solid solubility of carbon into iron is low.
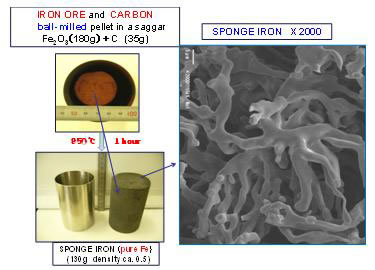
Figure 8
Experimental result showing the iron smelting at 950℃ by MA process applied to the raw materials (iron oxide and carbon).
6.2 Hydrogen generation from "water and iron" by MA
Referring to Fig.7 again, one may notice that the free energy of formation of H2O and FeO are quite close but iron oxide is slightly more stable than water throughout the wide temperature range including room temperature.
This fact means that pure iron can reduce water to produce hydrogen. We use iron and steel materials everyday without worrying much about the hydrogen generation due to water. This is because the interfacial area between iron materials and water is so small that the reactivity of iron and water can be thought negligible.
However, when the size of solid iron becomes very small as in the case of sponge iron we have seen in the previous experiment, the condition is entirely different. The sponge iron which are composed from micro-meter sized thin wires has specific surface area much larger than the iron rods of centimeter size.
Thus the sponge iron has the ideal form to be used as the hydrogen generation agent. The added advantage of iron to be used for this purpose is there are little heat generation in this reduction reaction because of the close oxidation potentials of iron and hydrogen as we confirmed in Fig.7
In reality, experiment proves that by simply immersing sponge iron into water produces no hydrogen gas(7). This is because of the existence of oxide layers which protect sponge iron to be attacked by water. The removal of such surface oxide layers by MA (mechanical grinding) has been proved to be quite effective as shown in Fig.9.
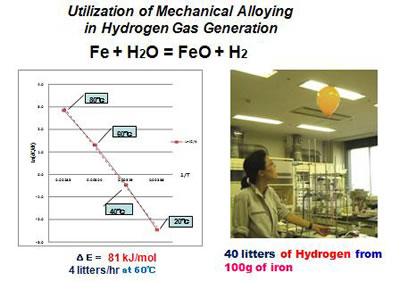
Figure 9
Experimental results of Hydrogen generation by MA of sponge iron and water
The result shown in Fig.9 is for the case of ball-milling of sponge iron with water, but additional experiments showed that once sponge iron is mechanically ground in dry inert atmosphere, the activity of hydrogen generation is maintained when the sample is sealed and stored in a bag filled with inert gas. By simply opening the bag and put the activated sponge iron into water, hydrogen comes out to the stoichiometric reaction limit (8,9).
Summary
Thus we have examined the potential of RQ, MA and related techniques in the production of meta-stable and unstable state of materials from both fundamental thermodynamic point of view and by experimental instances. These technologies when first found by experiments always looked somewhat unusual and strange but when examined and proved useful in years these become to be one of the conventional technologies.
When we look back such histories over RQ and MA, we realize that many seemingly "odd" behaviors of experimentalists and theorists may at times not odd at all ( Fig. 10 may be referred).

Figure 10
The Netherlandish Proverbs.
by Peter Beugel the Elder (1559).
Seemingly out of mind behaviors may sometimes not out of mind at all.
References
(1) J.W.Cahn: Proceedings of Second International Conference on Rapid Solidification Processing,
(1980) 24.
(2) P.H.Shingu and K.N.Ishihara: J. Alloys and Compounds, 194 (1993) 339.
(3) K.N.Ishihara, M.Maeda and P.H.Shingu: Acta.Met. Vol.33, No.12 (1985) 2113.
(4) D.Turnbull: Met. Trans. 12A (1981) 695.
(5) P.H.Shingu: "Kinzoku" vol. 71, No.1 (2001) 43.
(6) K.Yasuna, M.Terauchi, A.Otsuki, K.N.Ishihara and P.H.Shingu: J. Appl. Phys. 82(5), 1 Sept. (1997) 2435.
(7) P.H.Shingu: Bulletin of the Ceramic Society of Japan,44 (11), (2009) 838.
"Activation of iron by the mechanical alloying method: production of hydrogen from water and iron".
(8) P.H.Shingu: Japanese patent. Registration number, 5311334 (2013).
"Hydrogen production from sponge iron and water by MA".
(9) P.H.Shingu: Japanese patent. Registration number, 5413821 (2013).
"Low-temperature iron smelting utilizing MA".

Written by Shingu : November 17, 2016 03:26 PM